Laboratoire Léon Brillouin
UMR12 CEA-CNRS, Bât. 563 CEA Saclay
91191 Gif sur Yvette Cedex, France
+33-169085241 llb-sec@cea.fr
Laboratoire Léon Brillouin
UMR12 CEA-CNRS, Bât. 563 CEA Saclay
91191 Gif sur Yvette Cedex, France
+33-169085241 llb-sec@cea.fr
On Earth, water is abundant substance, the cycle of evaporation - condensation - solidification (steam transitions - liquid - solid) falls within everyday experience. The physical properties of water and its phase diagram are, however, much more complex than they appear with these familiar characteristics.
The properties of bulk water come from a delicate balance of interactions on length scales encompassing several orders of magnitudes: from the hydrogen bond at the intermolecular scale up to the hydrogen bond network at macroscopic level.
As can be deduced from figure (a) water molecules would like to form four equivalent so-called hydrogen bonds with their neighbors. The angles between these bonds should be close to 109 degrees like in a tetrahedron. But what happens to the dynamics of water when confined to a planar surface making the tetrahedral arrangement impossible. A team of scientists from CEA-IRAMIS in collaboration with the Synchrotron Soleil and the ILL has given this problem a closer look. They experimentally realized two-dimensional water by crafting the water molecules onto a hydrophilic surface with distances between the water molecules compatible with hydrogen bond formation (figure (b)). Understanding water confined to two dimensions is not a purely academic question. Water of this kind is ubiquitous in nature. A typical example is the hydration water on the surface of proteins.
An analytical mean-field percolation model approach provides a coherent interpretation of the different experimental observations detected in interfacial water at intermediate temperatures in the supercooled regime and below[1]. The important parameter of this statistical model is the probability p(T) that a water molecule forms a hydrogen bond with one of its neighbors. Neutron spectroscopy data are used to tune the temperature dependence of p(T).
What do we learn? At very low temperatures all water molecules are frozen within an amorphous ice state. As we increase the temperature several transitions are observed (see figure (c)). Starting at temperatures as low as 160 K water molecules are found capable of breaking hydrogen bonds. These mobile or liquid molecules have a strong tendency to cluster forming transient patches. These patches grow and at 220 K percolate, i.e. they form a connected surface. In the end a single patch emerges that covers the whole surface. The source of this particular behaviour in two dimensions is the frustration of the natural bulk tetrahedral local geometry and the underlying very significant increase in entropy of the interfacial water molecules.
The results of this study on the original structural and dynamic properties of two-dimensional water have relevance in all areas where water is in monolayer situation such as in the fields of biophysics and food industry or in materials and nuclear science (storage of long-lived waste).
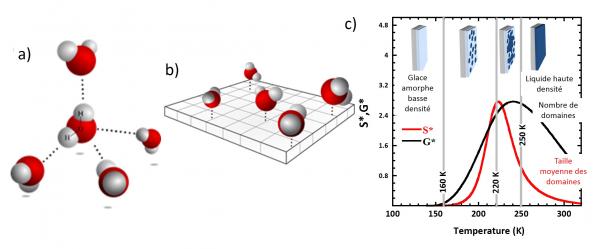
The ideal 2D tetrahedral organization of the network of hydrogen bonds (a) is frustrated (b). A molecule may only engage four hydrogen bonds: one with a silanol Si-OH group of the silica surface and the others with three neighboring water molecules. c) Water in 2D, a heterogeneous [1] system with unexpected physical properties: domains with high density interfacial waterarre fromed at the nucleation-growth transition at 165 K. Tg is the domain percolation transition at 220 K.
References:
| [1] Competing coexisting phases in 2D water J.-M. Zanotti, P. Judeinstein, S. Dalla-Bernardina, G. Creff, J.-B. Brubach, P. Roy, M. Bonetti, J. Ollivier, D. Sakellariou, and M.-C. Bellissent-Funel, Sci. Rep., 6, 25938 (2016). |
 |
|
[2] H. E. Stanley in Hydration process in Biology, M.C. Bellissent-Funel (Ed.), Nato Science Series – Serie A, vol 305, IOS Press (1999).
|
|
Contact CEA-IRAMIS: J.-M. Zanotti (LLB/Axe Soft Complex Matter / Groupe Spectroscopie)
ILL press release: "Competing coexisting phases in two-dimensional water".
Collaboration:
