Laboratoire Léon Brillouin
UMR12 CEA-CNRS, Bât. 563 CEA Saclay
91191 Gif sur Yvette Cedex, France
+33-169085241 llb-sec@cea.fr
Laboratoire Léon Brillouin
UMR12 CEA-CNRS, Bât. 563 CEA Saclay
91191 Gif sur Yvette Cedex, France
+33-169085241 llb-sec@cea.fr



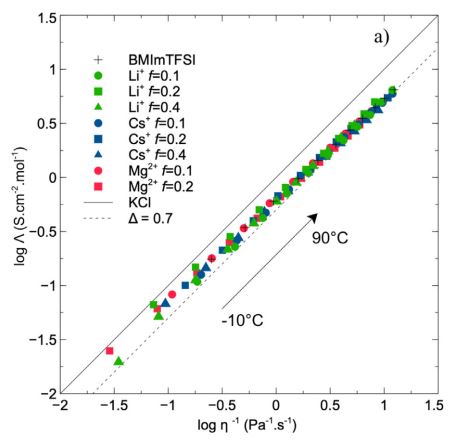
Properties of [X][BMIm][TFSI] electrolytes (X=Li, Cs, Mg): Walden plots combining viscosity and ionic conductivity in a large range of temperature.
H. P. Khanh Ngo, E. Planes, C. Iojoiu, P. Soudant, A.-L. Rollet, P. Judeinstein
The increasing need of portable electrical resources requires to develop post-Li batteries, in which redox reactions are then based on the different alkali or earth alkaline ions. Keeping in mind the specific advantages of electrolytes based on ionic liquid (electrochemical properties, safety, stability …), this paper focuses on the specific properties of those obtained by mixing alkali or earth alkaline salts (Li+, Na+, K+, Cs+ and Mg2+) in a prototypical ionic liquid, the 1-butyl-3-methyl imidazolium bis(trifluoromethyl sulfonyl) imide (BMImTFSI). Transport properties of these electrolytes are deciphered from viscosity, ionic conductivity and individual self-diffusion coefficients. At room temperature, change of electrolyte composition (nature of ion, concentration) induces some large variations of these transport properties. However, if these measurements are scaled towards glass transition temperatures, master curves are obtained with only slight differences between monovalent alkali and divalent earth alkaline ions. Further information is obtained from the Walden approach and evidences that these electrolytes are ’good ionic conductors’. These results are confirmed from self-diffusion coefficients which also allows to retrieve contributions of each species to ionic transport and then dissociation ratio inside these mixtures. Slight differences between alkali and earth alkali ions may be related to solvation mechanisms, as proposed from infrared spectroscopy measurements.
https://doi.org/10.1016/j.jil.2022.100044
•  Systèmes complexes et transition énergétique › Systèmes désordonnés et matériaux / Disordered systems, materials
Systèmes complexes et transition énergétique › Systèmes désordonnés et matériaux / Disordered systems, materials  Matériaux des nouvelles technologies pour l’énergie
Matériaux des nouvelles technologies pour l’énergie  Statistical physics and complex systems
Statistical physics and complex systems  Materials for new energy technologies
Materials for new energy technologies
• Laboratoire Léon Brillouin (LLB) • Leon Brillouin Laboratory (LLB)
• Groupe "Matière molle et biophysique" - MMB
• Spectrocopie nucléaires : RMN (Résonance Magnétique Nucléaire) - Spectroscopie Mössbauer
